有機合成化学、量子化学計算、時間分解レーザー分光を基盤として、高機能性フォトクロミック分子や有機ラジカルの開発を目指した物理有機化学の研究を行っています。
機能物質化学研究室

In the Functional Material Chemistry Laboratory, we are engaged in daily research activities with the aim of synthesizing unique molecules with unprecedented new functions and opening up the next frontier in materials science. In addition, we are actively promoting international joint research to develop practical human resources who can be active on a global scale.
◆ Laboratory Introduction Movie
◆ Media Coverage
・Precise molecular design using quantum chemical calculations
・Organic synthesis using various coupling reactions and functional group transformations
・Measurement of physical properties using state-of-the-art equipment
・Development of high performance photochromic molecules for light control materials
・Development and functional exploration of fast photochromic molecules
・Development of high-performance multifunctional photochromic molecules
・Precise control of electronic states of open-shell singlet biradicals
Key Word:Photochromism・Fast Photochromism・HABI・Singlet Biradical
The research proposal developed in our laboratory for the enhancement of the functionality of fast photochromic molecules was selected for the Japan Science and Technology Agency's Core Research for Evolutional Science and Technology (CREST) program from October 2010 to March 2016. We promoted research aimed at further improving the performance of fast photochromic molecules and exploring new functions. In addition, from July 2014, we participated as a core member for the MEXT's Grant-in-Aid for Scientific Research on Innovative Areas "Application of Cooperative-Excitation into Innovative Molecular Systems with High-Order Photofunctions", and worked on the Development of Photoresponsive Molecular Assemblies. In FY2018, we were selected for a Grant-in-Aid for Scientific Research (S) to develop nonlinear responsive photochromic molecules that respond to visible or near-infrared light.
Photochromism is a phenomenon in which a single chemical species reversibly produces two isomers of different colors without changing the molecular weight. When the isomer A is irradiated with light of a specific wavelength, it changes its bonding mode or electronic state and converts to the other isomer B, which has a different molecular structure, and as a result, the absorption spectrum changes and the color changes. The photogenerated isomer B returns to the original isomer A with the original molecular structure by light irradiation of a different wavelength or thermally, and the color also returns to the original. P-type photochromic molecules, such as diarylethene and fulgide, in which the isomer B is thermally stable, can remain in the isomer B state for a long time at room temperature unless exposed to light, whereas T-type photochromic molecule, such as azobenzene, spiropyran, or hexaarylbimidazole, the isomer B reverts to the isomer A by thermal reaction without light action. Today's thriving photochromism research originated from the study of spiropyrans by Fischer and Hirshberg in 1952. The T-type photochromic molecule, which reverts from the isomer B to the isomer A with thermal energy at room temperature, can switch the transmittance, refractive index, reflectance, and fluorescence intensity of a material simply by turning light irradiation on and off, making it suitable for a variety of applications. One of the most famous industrial applications is light modulating lenses. These are lenses for eyeglasses that reduce the glare of sunlight and protect the health of the eyes by lowering the transmittance of visible light through photoisomerization to the isomer B, which absorbs visible light in an environment with strong outdoor sunlight. Indoors, where the amount of ultraviolet rays is low, the color quickly fades back to the isomer A and becomes nearly colorless. Currently available light-controlling lenses require several minutes to several tens of minutes for complete fading, and research is underway to further speed up the thermal fading reaction. On the other hand, speeding up the thermal decolorization reaction rate of T-type photochromic molecules from milliseconds to nanoseconds will greatly expand the range of applications. For example, it is expected to be applied to all-optical logic circuits, super-resolution fluorescence microscopy, and real-time optical information processing.
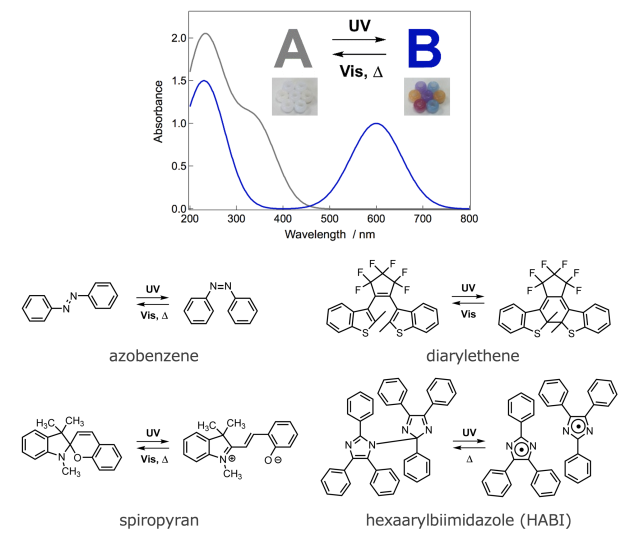
Absorption spectral changes associated with photochromism and typical photochromic molecules
Y. Inagaki, Y. Kobayashi, K. Mutoh, J. Abe
A simple and versatile strategy for rapid color fading and intense coloration of photochromic naphthopyran families
J. Am. Chem. Soc., 2017, 139(38), 13429-13441.
The imidazole dimer, hexaarylbiimidazole (HABI), is a domestically produced
photochromic compound that was accidentally discovered in 1960 by Prof.
Taro Hayashi and Dr. Koko Maeda at Ochanomizu University during their research
on chemiluminescence. In photochromism, the coloration reaction is the
generation of triphenylimidazolyl radicals (TPIRs) by photodissociation
of the C-N bond connecting the two imidazole rings, and the fading reaction
is the radical recombination reaction between the TPIRs generated by the
coloration reaction. The radical recombination reaction is a thermal reaction,
which is not accelerated by light irradiation, and is a typical T-type
photochromic molecule that quenches only by thermal reaction. The back
reaction from TPIR to HABI in solution is a thermal reaction that follows
a second-order reaction whose half-life depends on the concentration, so
it takes several minutes for TPIR to disappear the color completely.
Radicals with unpaired electrons are usually highly reactive and react
immediately with other chemical species to form stable molecules or ions.
For example, in proton-donating solvents such as alcohols, TPIRs are reduced
to rlophins by proton abstraction reactions from the solvent. Even in polymer
matrices, TPIRs produced by the light irradiation are gradually deactivated
by radical coupling reactions, making them unsuitable for light modulating
materials that reversibly change color in response to light. However, DuPont
has succeeded in commercializing a highly sensitive photopolymerization
initiator by utilizing the light-induced radicals of HABI. In particular,
Dylux is a typical photosensitive material that DuPont has successfully
commercialized after repeated improvement research and is applied to holograms
and photoresists. We are conducting basic research to clarify the properties
of HABI, which have not yet been clarified, by measuring the physical properties
of the newly synthesized HABI derivatives.
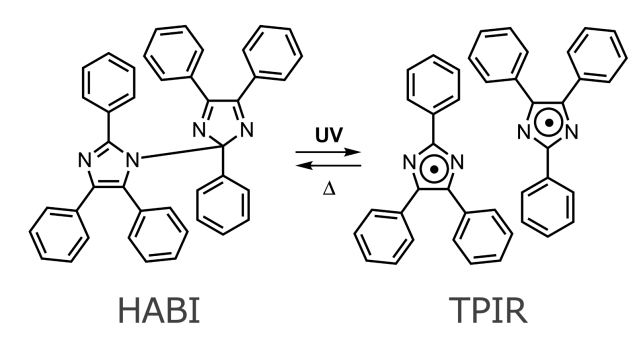
Photochromic reaction of HABI
In conventional HABI derivatives, TPIR, the colored isomer of the photochromic
reaction, dissipates in the medium, and the decoloration reaction, which
is a radical recombination reaction, is relatively slow. We were the first
in the world to create a bridged imidazole dimer that completely disappears
in a few hundred milliseconds at room temperature in solution. This fast
photochromic molecule is characterized by its combination of high coloration
efficiency and color concentration, which are not found in conventional
photochromic molecules, and fast thermal decolorization properties. In
other words, they exhibit " fast photochromism," which means
that they exhibit coloration only when irradiated with light and quickly
fade away when the light irradiation is stopped. The T-type photochromic
molecule, which can return from isomer B to isomer A with thermal energy
at around room temperature, can switch the transmittance, refractive index,
reflectance , and fluorescence intensity of a material just by turning
light on and off, making it suitable for a variety of applications. One
of the most famous industrial applications is photochromatic lenses. These
are lenses for eyeglasses that reduce the glare of sunlight and protect
the health of the eyes by lowering the transmittance of visible light through
photoisomerization to isomer B, which absorbs visible light in an environment
with strong outdoor sunlight. Indoors, where the amount of ultraviolet
rays is low, the color quickly fades back to isomer A and becomes nearly
colorless. Currently available light-controlling lenses require several
minutes to several tens of minutes for complete fading, and research is
underway to further speed up the thermal fading reaction. On the other
hand, speeding up the thermal decolorization reaction rate of T-type photochromic
molecules from milliseconds to nanoseconds will greatly expand the range
of applications. For example, it is expected to be applied to all-optical
logic circuits, super-resolution fluorescence microscopy, and real-time
optical information processing.
We are conducting research on innovative photochromic materials that specifically target practical high-speed photochromatic materials that have been difficult to realize with conventional photochromic molecules, real-time holographic materials for holographic 3D displays, high-speed photomechanical conversion materials that can be rapidly reversibly deformed by light stimulation, and high-speed photonics materials that can rapidly control the polarization plane and phase of light.
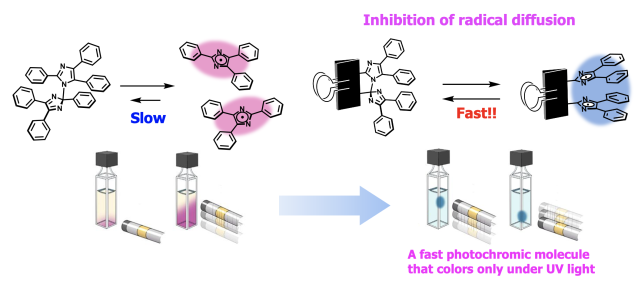
Concept of the bridged-imidazole dimer
Yuta Kishimoto and Jiro Abe
A fast photochromic molecule that colors only under UV light
J. Am. Chem. Soc., 2009, 131(12), 4227-4229.
Tetsuo Yamaguchi, Yoichi Kobayashi and Jiro Abe
Fast negative photochromism of 1,1’-binaphthyl-bridged phenoxyl-imidazolyl radical complex
J. Am. Chem. Soc., 2016, 138(3), 906-913.
Sayaka Hatano, Takeru Horino, Atsuhiro Tokita, Toyoji Oshima and Jiro Abe
Unusual negative photochromism via a short-lived imidazolyl radical of 1,1'-binaphthyl-bridged imidazole dimer
J. Am. Chem. Soc., 2013, 135(8), 3164-3172.
Norihito Ishii, Tetsuya Kato and Jiro Abe
A real-time dynamic holographic material using a fast photochromic molecule
Sci. Rep., 2012, 2, 819.
Norihito Ishii and Jiro Abe
Fast photochromism in polymer matrix with plasticizer and real-time dynamic holographic properties
Appl. Phys. Lett., 2013, 102, 163301.
Yoichi Kobayashi and Jiro Abe
Real-time dynamic hologram of a 3D object with fast photochromic molecules
Adv. Optical Mater., 2016, 4(9), 1354-1357.
Katsuya Mutoh,Yuki Nakagawa, Akira Sakamoto, Yoichi Kobayashi and Jiro Abe
Stepwise two-photon-gated photochemical reaction in photochromic [2.2]paracyclophane-bridged bis(imidazole dimer)
J. Am. Chem. Soc., 2015, 137(17), 5674-5677.
Izumi Yonekawa, Katsuya Mutoh, Yoichi Kobayashi and Jiro Abe
Intensity-dependent photoresponse of biphotochromic molecule composed of a negative and a positive photochromic unit
J. Am. Chem. Soc., 2018, 140(3), 1091-1097.
Katsuya Mutoh, Nanae Miyashita, Kaho Arai, Jiro Abe
Turn-on mode fluorescence switch by using negative photochromic imidazole dimer
J. Am. Chem. Soc., 2019, 141(14), 5650-5654.
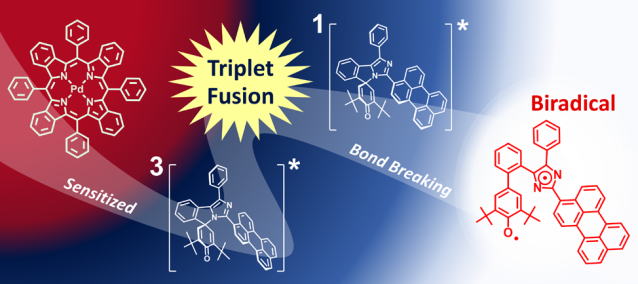
Ayako Tokunaga, Lucas Martinez Uriarte, Katsuya Mutoh, Eduard Fron, Johan Hofkens, Michel Sliwa and Jiro Abe
Photochromic reaction by red light via triplet fusion upconversion
J. Am. Chem. Soc., 2019, 141(44), 17744-17753.
Aya Kometani, Yuki Inagaki, Katsuya Mutoh and Jiro Abe
Red or near-infrared light operating negative photochromism of a binaphthyl-bridged imidazole dimer
J. Am. Chem. Soc., 2020, 142(17), 7995-8005.